Powered immersion corrosion is a critical challenge in today’s electronics, especially for smartphones, wearables, and devices exposed to moisture. We’ve all been through the morning bustle of a crowd when your arm gets bumped and coffee spills all over your new phone. You shake it off and rub the screen clean, but don’t notice the drop of liquid in the charging port. When you get in the car and plug in your phone to top off the charge, you accidentally set off a chain reaction of corrosion events. When liquids enter charging connectors, they can trigger an electrochemical reaction that rapidly corrodes contacts.
This blog explores the science behind powered immersion corrosion, how materials behave under these conditions, and how Xtalic’s advanced nanostructured alloys can provide long-lasting protection.
Contents
Understanding Powered Immersion Corrosion in Connectors
The connector on the phone, battery pack or smart watch, are highly engineered components designed to withstand thousands of mating cycles. The tiny metallic contacts make an electrical connection to the charging cord when you plug it in. Most lithium-ion batteries charge at a voltage of 4.2V and when the contacts are dry it isn’t a problem. However, if the contacts get wet, the liquids can act like an electrolyte, electrically bridging the gaps between the positive and negative electrical contacts to form an electrochemical cell. As the voltage is applied, the charging contacts quickly corrode. This can lead to early failure and an expensive repair.
The Science Behind Metal Dissolution Under Electrical Bias
The metal contacts used for charging are typically made of copper alloy then electroplated with various metals to provide stable performance. The metal coatings help lower contact resistance, provide durability and can prevent corrosion. However, not all metals perform the same in these tests.
To test the performance of metals under electrical bias, we can devise a simple electrochemical cell. Figure 1 shows the etch rates that occur in various metals when tested in artificial sweat under electrical bias. Au is the most common connector finish, but we can see from these data that gold begins to dissolve at around 2V. Most lithium-ion batteries charge at a voltage of 4.2V. At the recharge voltage, gold is readily dissolved.
Pure Ni begins to dissolve at around 2V and nanostructured Ni-W shows pitting corrosion at 3-3.5V. Platinum group metals, which more readily decompose water (into hydrogen and oxygen) instead of dissolving, can perform better. Palladium and its alloys are relatively stable at lithium ion recharge voltages and rhodium has excellent performance in this voltage regime.
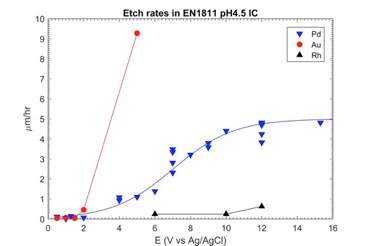
Figure 1. Shows the etch rates that occur in various metals when tested in artificial sweat under electrical bias.
Testing Methods for Powered Immersion Corrosion
Basic science tells us that material choice has an impact on final performance. To know which metal combinations will work best, we need to consider the end-use application and devise a test method to emulate those conditions. We know that the electrical connectors must survive both the electrochemical tests as well as a mechanical durability test. Each time we plug in the charging connector, the two metal surfaces slide over one another, causing some degree of wear at the mating interfaces. This wear can scratch through the thin, precious-metal outer layers, especially if they have been compromised by prior electrochemical attack.
The best test methods for this type of performance combine the mechanical durability aspects with some electrochemical testing. In some cases, we may perform 50 cycles of mechanical durability followed by a controlled drop of electrolyte into the connector and perform a simulated charge to drive the electrochemical reaction. This process can repeat many times until the connector eventually fails.
Figure 2. Adding a controlled drop of electrolyte into the connector interface.
Solutions to Combat Powered Immersion Corrosion
Test data and a decade of high-volume manufacturing show that a combination of corrosion-resistant finish layer with a hard and durable underlayer promotes the best performance and value in a connector exposed to powered immersion corrosion. The top layer should be a water-splitting capable metal, such as rhodium, platinum or PALLEX® (our own nanostructured Pd alloy. The underlayer supports the top layer for wear and corrosion, nanocrystalline Ni-W layer, XTRONIC® is an ideal choice. XTRONIC is durable and the corrosion mechanism provides for longer-lasting performance in powered immersion corrosion.
Applications of Powered Immersion Corrosion-Resistant Coatings
Since 2015 Xtalic has protected billions of USB-C and similar charging connectors using our technology. Consumer electronics can be exposed to challenging environments and benefit from the protection these solutions provide.
Military and defense applications also show compelling value propositions as these products often operate in extremely harsh environments and require mission critical reliability.

Smart watches include charging pads which regularly are exposed to perspiration and other liquids which could degrade the performance of the charging interface.

By leveraging the strengths of materials science in powered immersion corrosion tests, we can boost the performance of these connectors reducing the overall cost of ownership.





