Contents
Integrating Corrosion Resistant Alloys for Faster Smartphone Charging Connectors
Since their first real popularization in the mid-2000s, smartphones have enabled information sharing and addictive use patterns. We love our phones and carry them everywhere. As devices get more powerful and we find more ways to use them, the engineering required to keep them operating well becomes more complex. It is summertime where I live, and that means my smartphone went with me on vacation – the beach and the ocean and air travel are great but introduce opportunities for my phone to get exposed to salt water and other corrosive media. Jim Yurko, Sr. Director of Materials Engineering at Apple, highlighted this risk during his keynote lecture at the MS&T 2021 TMS Materials Science Conference. There, he shared this humorous commercial of an overzealous amateur chef spilling and splashing all over his phone while vigorously preparing a meal.
Most phones protect their valuable and sensitive components with rugged enclosures, robust toughened glass, sealed speakers and microphones, or a protective cover. But the charging port remains a vulnerability to corrosive attack. To effectively transmit electrons, allowing charge and data transfer, the metallic electrical contacts need to be directly exposed to the user and hence have some risk of corrosion.
In the beginning, charging powers were low, and it was less of a problem. However, we have now advanced to USB-C type fast charging interfaces. These give us a big boost of charge in a short amount of time but require more power, sometimes up to 20 Watts, and power can accelerate corrosion.
Technology Options for Resisting Corrosion on Smartphone Charging Connectors
Covers
Some designers tried elastomeric flaps to cover the charging port, relying on the user to close the flap and provide protection. Elastomers are durable and easy to form and can be made visually attractive, but how does a phone company manage a warranty return for a corroded charge port when the user fails to employ the cover adequately?
Electronic Controls
When a drop of sweat is inside the charging port, there is an electrical pathway between the adjacent contacts through the drop of sweat. It is possible for the charging hardware and software to scan the contact-to-contact voltages and resistances to determine if a foreign body is in the charging interface. If the system detects a drop of sweat as a non-open circuit resistance between two contacts, it can prevent the phone from charging. This protects the phone from an electrochemical attack but also prevents the phone from charging until the fault is cleared.
Wireless Charging
This remains an option that would eliminate the charging I/O port. Wireless data transfer is generally available, and audio output can be all Bluetooth based. The phone could be completely sealed and made more robust with sufficient wireless power charging rates. However, wireless charging remains slower, less efficient, and hotter than I/O port charging. More on this in a future post.
Engineering the I/O port
Using computational materials science, we can engineer the charge port interface to improve the corrosion resistance of smartphone connectors. Gold and nickel both readily electrochemically dissolve in salt water at the voltages typical of mobile phone charging. By engineering a stack of coatings on the charging interface, we can tailor the corrosion resistance performance to optimize it for durability and cost. Some of the best-plated stacks in smartphones today start with corrosion resistant alloys made up of a layer of smoothing copper, then add a hard, durable, and corrosion-resistant layer of nanocrystalline nickel-tungsten, followed by layers of palladium and a top coat of platinum or rhodium.
The top coats of platinum and rhodium are particularly adept at splitting water into its elemental components of hydrogen and oxygen when exposed to water under an electrochemical electrical bias. As such, during a charge event, the applied voltage in the charger acts to split the water into its gaseous components, which quickly and harmlessly emanate from the interface. The nanocrystalline nickel layer supports the precious metals during the mechanical challenge of multiple mate/unmate cycles.
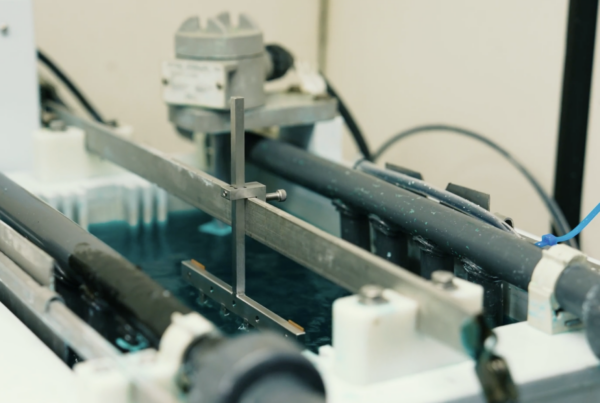
The need for a strong and durable underlayer became more evident as we partnered with phone OEMs to create new test methods for this emerging technology. While exposure to sweat as a corrosive media was a suitable and realistic accelerant, we also need to incorporate the impact that mechanical mating would have on the contact interface. The electrical contacts in the charge port can be as thin as knife blades and scrape across each other during each mating cycle. This action can scratch and damage the thin coatings used to protect the delicate contacts. Most consumer electronics companies now include both mechanical mating and electrochemical testing as part of the qualification protocol.
By leveraging knowledge of materials science and stack development for corrosion protection, Xtalic worked with the industry to create test standards and technology approaches for waterproof smartphones that reduce customer warranty returns and therefore lowering the overall cost to the consumer. As an engineer working on these devices, and more importantly, as a user of them, I am grateful for the improved durability that enables us to take our phones to the beach and charge them in less than half the time it used to take.